Research
Embryonic myeloid cells, especially macrophages, have remarkable functional diversity:
- innate immune responses
- tissue development
- tissue homeostasis
Tissue-resident macrophages are present in nearly all tissues of the body. In adults, they are derived from several different developmental origins and display distinct phenotypes and gene expression profiles.
Despite extensive studies on the origin, kinetics, and characteristics of embryonic macrophages, only a few molecules involved in embryonic leukocyte migration are known.
Right now, we are intrigued with immune-endocrine interaction and how myeloid cells could modulate endocrine cell development, hormonal signaling, and metabolism. Immune-endocrine interaction could extend its effects on the metabolism, survival, and reproduction of an individual.
In addition, macrophages have been linked to several human diseases from cancer to metabolic disorders. However, our understanding of how different macrophage subsets function under disease conditions is still limited. Our research aims to pinpoint the differences between embryonic and adult-derived macrophages to be utilized in more specified and punctual treatment options in the future.
We utilize multi-parameter cytometry and single-cell RNA sequencing, as well as different advanced imaging methods in our work. With various genetic in vivo models and lineage tracing methods, we follow the fate of individual cells and their progeny.
Projects
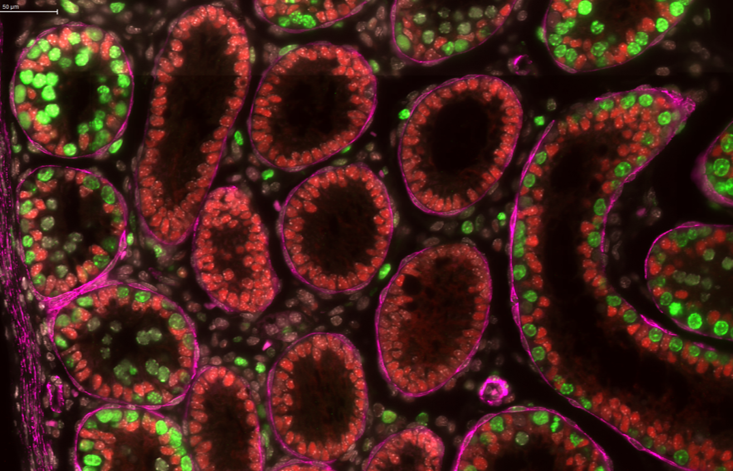
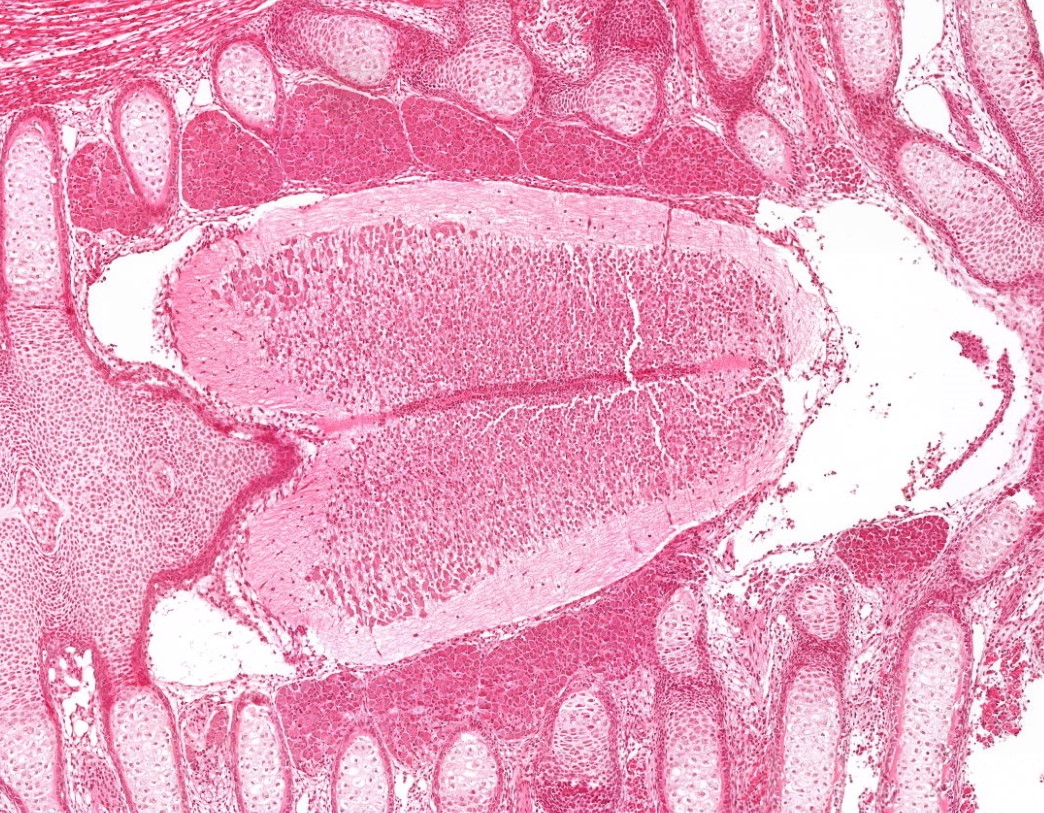
Generation, localization, and functions of myeloid cells in testis

Embryonic-derived macrophages in brown adipose tissue development and function

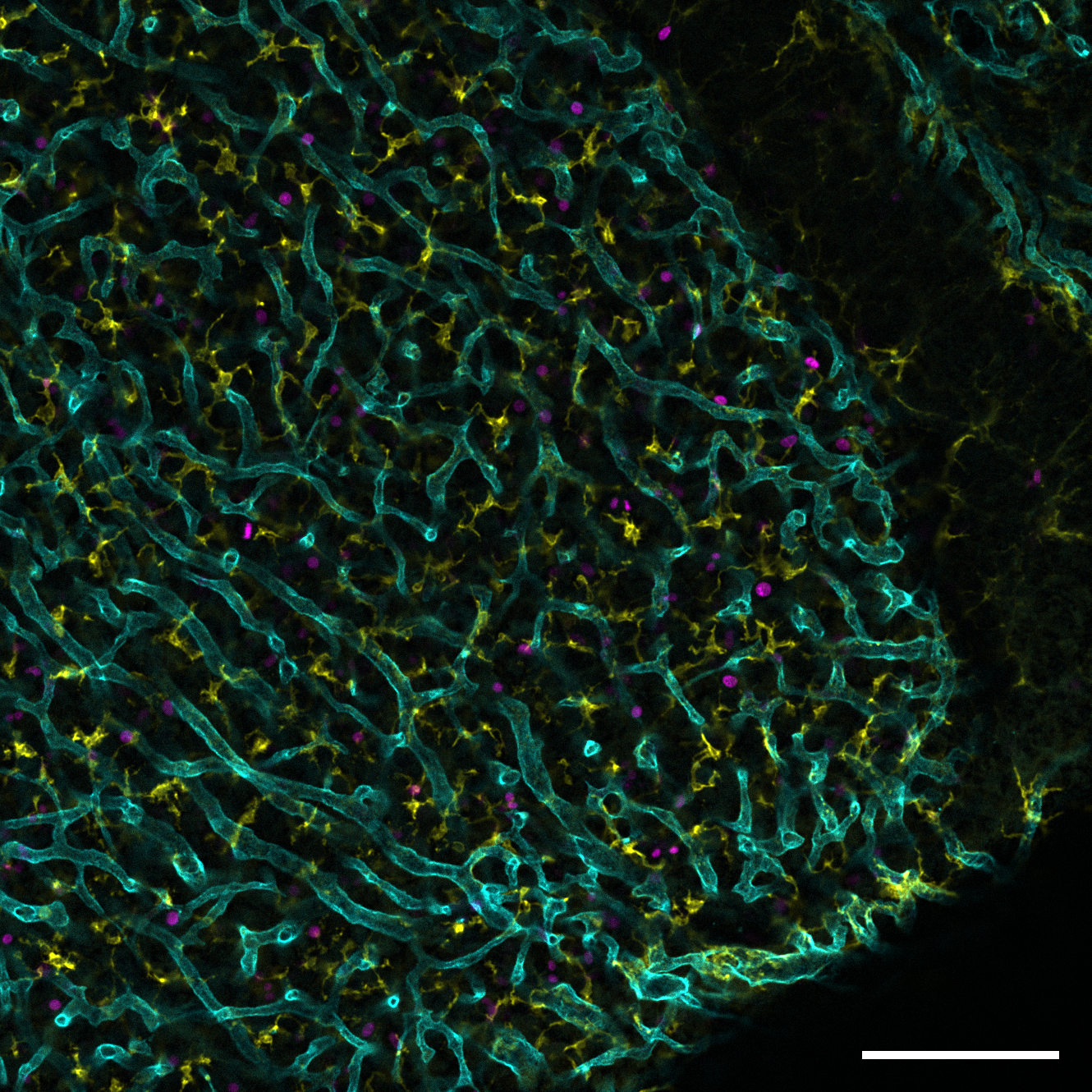
Pituitary gland myeloid cell populations and their impact on hormonal regulation.
The first part of the project on “Early precursor-derived pituitary gland tissue-resident macrophages play a pivotal role in modulating hormonal balance” in now out in Cell Reports!